Marine battery maintenance tips
Industry experts say that excessive sulfation can stop batteries from working long before their internal components wear out.
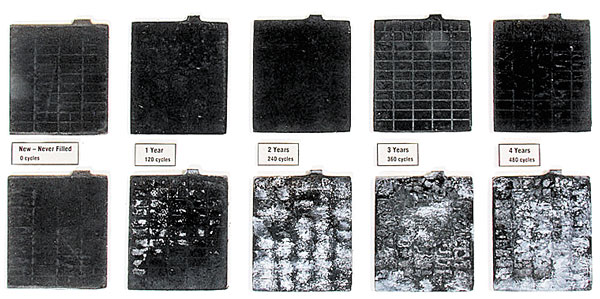
The comparative images published here are the most graphic evidence I have seen regarding the damage that excessive plate sulfation can do to a battery. These images were generated during a test in which a PulseTech Products dealer in Minnesota simulated four years of discharging and recharging two sets of batteries, one set with a very good conventional charger and one with a charger including pulse technology.
What is sulfation?
The sulfuric acid in a battery’s electrolyte combines chemically with the lead in the battery’s plates to produce electrical power. This chemical process causes a spongy coating of lead sulfate to form on the plates. There is nothing wrong with this; it has to happen as a normal part of a battery’s discharge process during everyday use. Once all of the available acid in the electrolyte combines with all the available plate surface area, the chemical reaction stops and the battery ceases to produce power.
Promptly recharging the battery dissolves almost all of the spongy coating, and “promptly” is the key word here. The spongy coating hardens into a crystalline structure over time and the longer the battery is left discharged, the more difficult the coating is to remove. If delayed recharging becomes typical, slowly spreading “scabs” of these crystals gradually cover more plate surface area, insulating it from the electrolyte, and reduce the battery’s storage capacity until there isn’t any left.
Simply put, less available plate area means less plate contact with the acid in the electrolyte and less total power available between recharges.
Damage control
Recharging a battery immediately after use and keeping it at least 75 percent charged while it is waiting to be used again minimizes sulfation, but, as a recent test showed, it doesn’t always completely eliminate it when using a conventional charger. The general assumption among users of pulse-charging technology has been that, as charger manufacturers claim, it reduces sulfation and increases the service life of batteries. The problem is, regardless of what anyone claimed, most of us had to use a charger with pulse technology over a period of years to prove it to ourselves.
PulseTech Products of Southlake, Texas, (pulsetech.net, 800-580-7554) has been marketing chargers with pulse technology for nearly two decades, and one of the company’s Minnesota dealers recently ran a test over an eight-month period to illustrate its value. The test consisted of discharging and recharging two groups of batteries, recharging one group with pulse technology and one without it.
The test was conducted with the assumption that the average battery sees 120 discharge/recharge cycles per year. A total of 480 cycles, the equivalent of four years of service, were accomplished by charging each battery to a full charge of 12.8 volts and then discharging it with a five-amp draw down to 12.1 volts.
These images of the battery plates were recorded in 120 cycle increments to show the amount of sulfation each battery accumulated in the equivalent of a year’s use. The top row of plates were pulse-charged with a PulseTech XC-100 charger with a 2.5-amp actual (5-amp effective) rate, and the bottom row was charged with a conventional transformer-based charger rated at 4 amps. I found the images startling.
So how does pulse charging work? The technical answer from PulseTech is that the technology’s effectiveness comes from the pulse waveform the company uses. The pulse includes the patented combination of a strictly controlled rise time, pulse width, frequency and amplitude of current and voltage, all designed to remove excess lead sulfate deposits from battery plates and convert them back into active electrolyte.
When used regularly, a pulse charging system will also help prevent the lead sulfates from building up again. The technology works well enough for the company to sell commercial grade, HD Xtreme Recovery Chargers capable of bringing sulfated-to-death batteries back to life. The company estimates that more than 80 percent of batteries are ruined by sulfation long before they die of old age, and pulse charging can make a battery last up to three times longer than one that gets just average care.
The bottom line
Recharging your boat’s batteries promptly after a day on the water or your camper’s batteries as soon as possible after you run them down will minimize the formation of permanent lead sulfate crystals no matter which type of charger you use. Using a pulse charger can remove virtually all of them and prevent future problems. Pulse charging can’t fix mechanical problems, like warped plates or internal shorts, but it can keep the permanent formation of lead sulfate on plates to a bare minimum, ensuring that you get all the power you paid for from your batteries between recharges until they actually do wear out.
My last set of Rolls deep-cycle batteries lasted nine years without pulse technology. I’m now running a set of PulseTech’s XtremeCharge units to maintain my boat’s three batteries between fishing trips along with the company’s Solar Pulse solar panel units to pulse the batteries while I’m out on the water.
Now that gas prices are ridiculously high again, my truck sits until I need to haul something or pull the camper or boat. I keep an XtremeCharge unit on it while it sits. I can’t wait to see how long this set of marine batteries will last, but I guess I will have to.


